Dao Duy Quang, Truong Dinh Hieu, Pham Le Minh Thong, Dinh Tuan, Pham Cam Nam, Ime Bassey Obot. DFT study of the interactions between thiophene-based corrosion inhibitors and an Fe-4 cluster. Journal of Molecular Modeling, 23(9), 260. (ISI, IF = 1.425)
Ngày: 19/12/2018
Understanding the physicochemical properties of corrosion inhibitors and their chemical interactions with metal surfaces is crucial to the design of improved (i.e., more efficient) corrosion inhibitors. In this work, the physicochemical properties of six thiophene-based corrosion inhibitors (2-acetylthiophene (AT), 2-formylthiophene (FT), thiophene (Th), 2-methyl-3-thiophenthiol (MTT), 2-pentylthiophene (PT), and 2-thenylthiol (TT)) were systematically studied by performing ab initio calculations at the MP2(full)/6-31G(2df,p) level of theory. Adsorption of the inhibitors on an iron surface was also modeled by investigating the interactions of these molecules with a tetrahedral Fe4 cluster using the B3LYP method and the 6-311G(d,p) basis set or the LanL2DZ basis set. The calculated results indicate that the nature of the substituent group present has a significant impact on the geometric and electronic structures of the thiophene-based molecules. The presence of an electron-donating group causes the electron density in the thiophene ring to increase, while the presence of an electron-withdrawing group has the opposite effect. Accordingly, the examined molecules were ranked in order of corrosion inhibition efficiency as follows: FT ≈ AT < Th < PT < TT < MTT. The calculated binding energies demonstrated that the π−1Fe and π−3Fe interaction configurations dominated over the S−1Fe configuration for all the compounds. Natural bond orbital analysis revealed that all of the thiophene-based compounds donate electrons from the π and σ orbitals of high-electron-density regions such as C2−S1−C5 and C3−C4 or from two lone pairs on S1 to the Fe4 cluster. Although electron donation from the thiophene-based compounds is always the dominant electron transfer process during adsorption, the backdonation of electrons from the 3d orbital of iron to σ*-antibonding orbitals of the thiophene compounds is also observed, especially in the case of π−3Fe parallel adsorption.
Graphical abstract
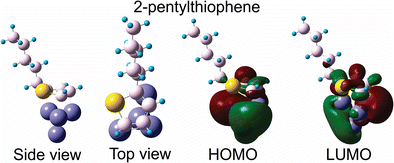
Optimized geometry, HOMO and LUMO for the π–3Fe interaction configuration of 2-pentylthiophene and Fe4 cluster
Thiophene Inhibitor Iron Corrosion Adsorption DFT NBO
Bài viết liên quan